
Adgyl Lifesciences offers full range of Safety Assessment Regulatory Tox, IND enabling CMC and PharmTox packages. It also offers both cGMP material for your early clinical development, late clinical development (for niche molecules).
We have successfully submitted 90+ IND packages for submission to regulatory agencies across the world including USEPA, Mexico, Brazil, USFDA, ICH, MHA and EMA. Adgyl is a GLP and AAALAC-accredited lab and was successfully audited by the USFDA (2012 and 2016) for GLP studies submitted as part of IND packages.
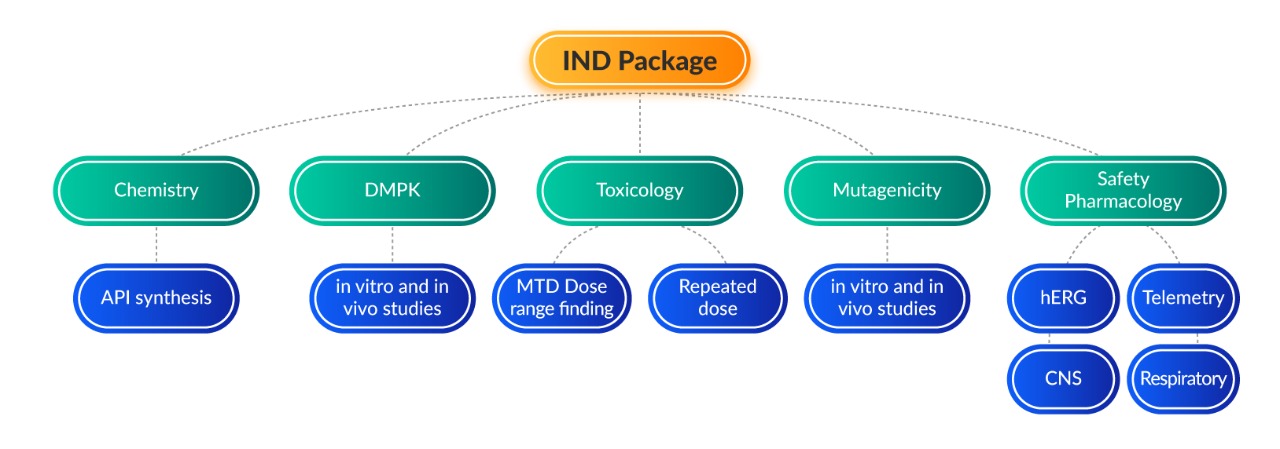
Exploratory studies (Non-GLP)
Genotoxicity (GLP)
Safety Pharmacology (GLP)
Toxicology studies (GLP)