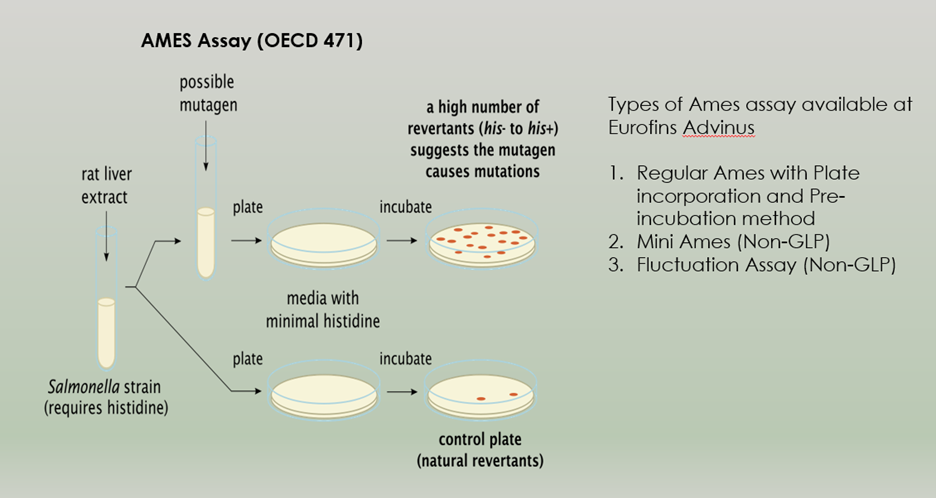
Genetic Toxicology is the study of genetic damage to the hereditary material that results in genetic alterations. Furthermore, Genetic Toxicology studies are conducted to assess the potential for induction of genetic mutations or chromosomal damage, by identifying substances that can cause genetic alterations in somatic and/or germ cells. Additionally, regulatory decisions utilize this information. Genetic toxicity testing is, therefore, an important component of a complete safety assessment profile for all new products (e.g., pharmaceutical ingredients, pharmaceutical impurities, agrochemicals, pesticide plant residues/metabolites, industrial chemicals, etc.).
Adgyl Lifesciences offers Genetic Toxicology Studies in accordance with OECD and ICH guidelines and in compliance with GLP. The Adgyl Lifesciences team has completed more than 2000 studies over the past 3 decades and successfully submitted this data to a variety of regulatory authorities worldwide. Moreover, the company assists sponsors in developing testing strategies for conducting regulatory studies.